Multiple Choice
Identify the
choice that best completes the statement or answers the question.
|
|
|
|
|
|
1.
|
Based on the periodic table shown, elements from columns B and E will combine in
which ratio?
a. | 2B:3E | c. | 3B:2E | b. | 3B:1E | d. | These elements will not
combine. |
|
|
|
2.
|
Group 13 elements tend to acquire which charge when they form ions?
|
|
|
3.
|
How many total electrons must be transferred to form one formula unit of
the compound Al2O3?
|
|
|
4.
|
Which is a correct statement of bond strength?
a. | Compounds with smaller atoms have weaker bond strength. | b. | Compounds with a
higher total number of atoms in the compound have greater bond strength. | c. | Compounds containing
atoms with greater charges have higher bond strength. | d. | Compounds with a more negative lattice energy
have lower bond strength. |
|
|
|
5.
|
Which is the correct name for the compound CaClO2?
a. | Calcium Chloride | c. | Calcium Chlorite | b. | Calcium Chloroxide | d. | Calcium
Chlorate |
|
|
|
6.
|
Which combination of bonds is present in this molecule?
O = O
a. | 1 sigma bond only | c. | 2 pi bonds | b. | 1 sigma bond and 1 pi bond | d. | 1 sigma bond and 2 pi
bonds |
|
|
|
7.
|
Which exception to the octet rule is shown by this molecule? 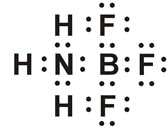 a. | uneven number of valence electrons | c. | expanded octet | b. | suboctet | d. | coordinate covalent bond |
|
|
|
8.
|
How many single covalent bonds can halogens form?
|
|
|
9.
|
How many valence electrons are available in the ion
NO3-?
|
|
|
10.
|
How many pi bonds are there in a triple covalent bond?
|
|
|
11.
|
What is the correct ratio of coefficients to balance this chemical
equation? 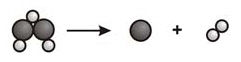 a. | 1:2:3 | c. | 2:4:3 | b. | 1:2:1.5 | d. | 1:1:2 |
|
|
|
12.
|
How many total atoms are in 3Na2SO4?
|
|
|
13.
|
Which type of reaction involves one element and one compound reacting?
a. | decomposition | c. | single replacement | b. | double replacement | d. | synthesis |
|
|
|
14.
|
Which substances have the same empirical formula? | Sample | Formula | | 1 | CH3OH | | 2 | CH2O | | 3 | C6H12O6 | | 4 | C2H4O2 | | 5 | C7H5O2 | | 6 | C8H8 | | |
a. | Samples 1, 2, and 4 | c. | Samples 1 and 3 | b. | Sample 1 and 4 | d. | Samples 2 and 3 |
|
|
|
15.
|
Which is the correct empirical formula for this substance? | Element | Percent Composition | | C | 37.5% | | H | 12.5% | | O | 50.00% | | |
a. | CH4O | c. | C3H12O3 | b. | CHO | d. | C3H4O |
|
|
|
16.
|
Which is the percent composition of bromine in the compound NaBr?
a. | 81.6% | c. | 84.1% | b. | 79.9% | d. | 77.7% |
|
|
|
17.
|
Which term is described as the percent by mass of any element in a
compound?
a. | hydrate | c. | empirical formula | b. | molecular formula | d. | percent
composition |
|
|
|
18.
|
Which is true of the reaction shown below? 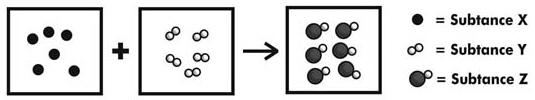 a. | The mole ratio of this reaction is 6:5:6. | b. | Two molecules of
Substance Y will be left over when this reaction goes to completion. | c. | Substance Y is the
limiting reagent in this reaction. | d. | The addition of more molecules of Substance X
will not affect the amount of Substance Z that can be made. |
|
|
|
19.
|
How many moles of Cu are needed to react with 5.8 moles of
AgNO3?
Cu + 2 AgNO3 ®
Cu(NO3)2 + 2 Ag
a. | 2.9 moles | c. | 5.8 moles | b. | 3.8 moles | d. | 11.6 moles |
|
|
|
20.
|
A reaction was predicted to produce 32.4 grams of a compound. When the product
was measured, there were only 26.1 grams made. What is the percent yield of this reaction?
a. | 80.6% | c. | 24.1% | b. | 6.3% | d. | 58.5% |
|
|
|
21.
|
How many grams of Fe 3O 4 are required to react completely
with 300 grams of H 2? 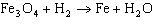 a. | 2096 g | c. | 8681 g | b. | 1.54 g | d. | 37.5 g |
|
|
|
22.
|
What principle is illustrated in the figure? 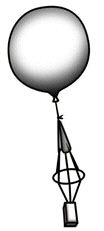 a. | Boyle’s Law | c. | Ideal Gas Law | b. | Charles’s Law | d. | Scientific
Theory |
|
|
|
23.
|
According to Gay-Lussac’s law:
a. | pressure is inversely proportional to volume at constant
temperature. | b. | pressure is directly proportional to temperature at constant
volume. | c. | volume is inversely proportional to temperature at constant
pressure. | d. | volume is directly proportional to temperature at constant
pressure. |
|
|
|
24.
|
When a milkshake is taken in through a straw at a pressure of 0.071 atm, the
straw contains 5.0 mL of liquid. How much liquid is consumed at 0.092 atm?
a. | 0.10 mL | c. | 6.3 mL | b. | 3.9 mL | d. | 7.8 mL |
|
|
|
25.
|
A welding torch requires 3200 L of ethylene gas at 3.00 atm. What will be the
pressure of the gas if ethylene is supplied by a 250.0-L tank?
a. | 0.231 atm | c. | 38.4 atm | b. | 2.34 atm | d. | 45.4 atm |
|
|
|
26.
|
A/An _____ is a substance that slows down the rate of a reaction.
a. | catalyst | c. | reactant | b. | inhibitor | d. | product |
|
|
|
27.
|
If a collision between molecules is very gentle, the molecules are
a. | more likely to be oriented favorably. | b. | less likely to be oriented
favorably. | c. | likely to react. | d. | likely to rebound without
reacting. |
|
|
|
28.
|
A _____ is produced when a base accepts a hydrogen ion from an acid.
a. | conjugate acid | c. | acid | b. | conjugate base | d. | base |
|
|
|
29.
|
In the Bronsted-Lowry model of acids and bases, an _____ is a hydrogen donor and
a _____ is a hydrogen acceptor.
a. | acid, base | c. | conjugate acid, conjugate base | b. | base,
acid | d. | conjugate base,
conjugate acid |
|
|
|
30.
|
A solution that contains equal concentrations of hydrogen and hydroxide ions is
_____.
a. | an acid | c. | neutral | b. | a base | d. | ionized |
|
|
|
31.
|
Calculate the hydrogen ion concentration of an aqueous solution, given the pOH
of the solution is 4.50 and the ion product constant for water, KW, is 1.00 ´ 10–14.
a. | 3.16 ´ 10–10 M | c. | 3.16 ´ 10–5 M | b. | 3.16 ´ 10–9 M | d. | 3.16 ´
10–7 M |
|
|
|
32.
|
An aqueous solution has a pH of 2.7 at 298 K. Calculate the pOH of the aqueous
solution.
|
|
|
33.
|
What is the oxidation number of chlorine in NaCl?
|
|
|
34.
|
_____ numbers are based on the distribution of electrons in a molecule.
a. | coefficient | c. | electron | b. | oxidation | d. | chemical |
|
|
|
35.
|
_____A species whose oxidation number _____ is oxidized.
a. | increases | c. | stays the same | b. | decreases | d. | is neutral |
|
|
|
36.
|
Calculate the hydrogen ion concentration of an aqueous solution, given the
concentration of hydroxide ions is 1 ´ 10-5 M and the ion
constant for water is 1 ´ 10-14.
|
|
|
37.
|
When acids react with metals, they produce _____ gas.
a. | hydrogen | c. | sulfur | b. | nitrogen | d. | oxygen |
|
|
|
38.
|
Calculate pH of an aqueous solution of hydrogen chloride acid. Given the
hydrogen ion concentration is 8.75 ´ 10–9
M.
|
|
|
39.
|
Calculate the pOH of a 0.410 M Ba(OH) 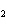
solution.
|
Short Answer
|
|
|
40.
|
Elements in groups 1A and 2A in the periodic table form positively charged ions
by loss of electrons. What will be the charge on an atom, if it belongs to group 1A?
|
|
|
41.
|
Which kind of molecular shape will this molecule have? Explain how you can tell
from the Lewis structure. 
|
|
|
42.
|
Why is it necessary to use prefixes in naming covalent compounds?
|
|
|
43.
|
Infer three pieces of evidence that a chemical reaction is taking place in the
pictures shown. 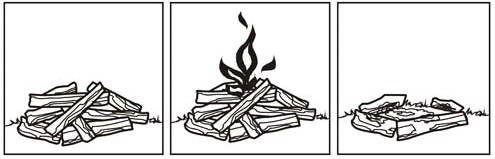
|
|
|
44.
|
Fill in the missing conversion factors in the diagram. 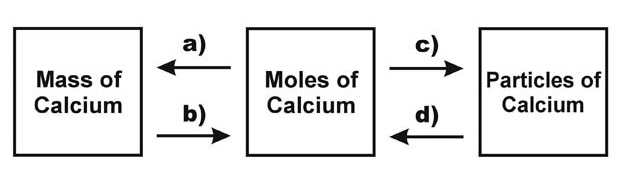
|
|
|
45.
|
When converting a temperature from Celsius degrees to Kelvin degrees, what
number must be added to the Celsius temperature?
|
Completion
Complete each
statement.
|
|
|
46.
|
An ionic bond results due to the ____________________ attraction between two
oppositely charged ions.
|
|
|
47.
|
The name of the anion 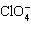 is
____________________.
|
|
|
48.
|
The name of the acid HI is ____________________ acid.
|
|
|
49.
|
A chemical reaction in which oxygen combines with a substance and releases
energy in the form of heat and light is called a(n) ____________________ reaction.
|
|
|
50.
|
A reaction in which the atoms of one element replace the atoms of another
element in a compound is called a(n) ____________________ reaction.
|
|
|
51.
|
In an aqueous solution, the solvent is always ____________________.
|
|
|
52.
|
The molar mass of carbon tetrachloride is ____________________ g/mol.
|
|
|
53.
|
A compound that has specific number of water molecules bound to its atoms is
known as a(n) ____________________.
|
|
|
54.
|
A ____________________ is the species produced when a base accepts a hydrogen
ion from an acid.
|
|
|
55.
|
In the ____________________ model of acids and bases, an acid is a hydrogen-ion
donor and a base is a hydrogen-ion acceptor.
|
|
|
56.
|
When an atom ____________________ electrons, its oxidation number
decreases.
|
|
|
57.
|
In chemistry, a ____________________ is any kind of chemical unit involved in a
process.
|
|
|
58.
|
|
|
|
59.
|
The ____________________ of a reaction is defined as the sum of powers of the
concentration in the rate law.
|
|
|
60.
|
In the reaction, 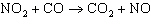 , the mechanism is: 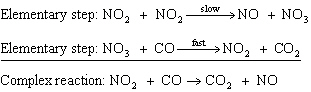 The intermediate compound for the mechanism above is ____________________.
|
Problem
|
|
|
61.
|
Why is PCl 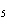 an exception to the octet rule, while PCl 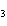 is not?
|
|
|
62.
|
What is the percent yield for a reaction if the theoretical yield of
C6H12 is 21 g and the actual yield recovered is only 3.8 g?
|
|
|
63.
|
A 6.32-L football is filled with air at 1.90 atm at 25.1°C. At the same
temperature, the volume of the football is reduced to 3.49 L. What is the pressure of air in the
ball?
|
|
|
64.
|
Calculate the temperature of 2.0 moles of a gas occupying a volume of 5.0 L at
2.46 atm.
|
|
|
65.
|
When nitrogen gas reacts with hydrogen gas, ammonia gas is formed. How many
grams of hydrogen gas are required to react completely with 3.5 L of nitrogen at STP?
|
|
|
66.
|
If the pressure exerted by a gas at 27.0°C in a vessel of volume 0.050 L is
4.00 atm, how many moles of the gas are present?
|
|
|
67.
|
Calculate the volume of the vessel that holds 0.30 moles of a gas at STP.
|
|
|
68.
|
For a hypothetical reaction, 1.2A + 2.5K + 4.4X, give the rate law expression
and calculate the order of the reaction.
|
|
|
69.
|
Determine the net change of the oxidation number of silver in the following
redox reaction, given the oxidation number of sulfur in Silver sulfide is –2. Silver +
Sulfur  Silver sulfide
|
|
|
70.
|
When lead sulfide reacts with oxygen, the precipitate of lead oxide is formed
and sulfur dioxide gas is evolved. Balance the redox equation by the half-reaction method. PbS +
O 2  PbO + SO 2The net ionic
equation for the redox reaction is S 2– + O 2 
SO 2 + O 2–
|