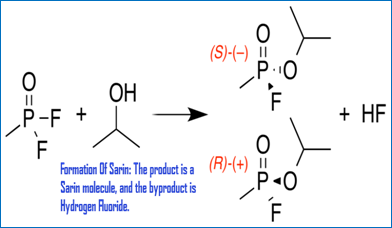
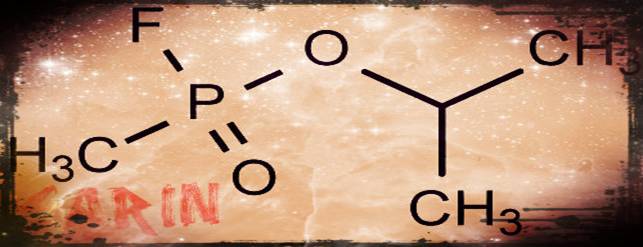
Sarin Gas: Pesticide to chemical weapon by Christoper Cassetta
Sarin use in Syria by Victora Boller
Sarin Gas: Should we get involved? By Kristen Daniel
Sarin Gas: A danger to Society! by Sarah Parker
Sarin Gas and War by Jeremy Smith
Crime Science
In this experiment, the student observed reactions that occur to identify two unknowns. The known solutions are mixed with each of three different reagents. Using these reactions as a reference the student developed a systematic set of tests to identify the unknown solutions.
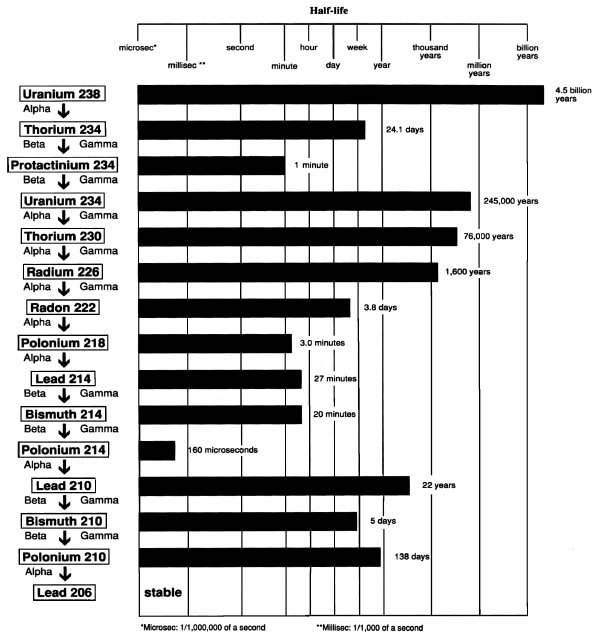
Fukushima: Nuclear Disaster
By: Margaret McCarthy
The Fukushima Disaster
By Ben Greep
Fukushima Expository Paper
By Jenny Daily
Fukushima Nuclear Disaster
by Ryan Hay
The Great Eastern Earthquake
by Aditya Ponugupaty
The Great Eastern earthquake and the tsunami that occurred on March 11, 2011 caused the meltdown of three nuclear reactors within several days, and four hydrogen explosions. Huge quantities of radioactive elements have been continuously released into the air and water. It will induce an epidemic of cancer, as people inhale the radioactive elements, eat radioactive vegetables, rice, and meat, and drink radioactive milk and teas.
Magnesium burns bright in color and the ash weights more than the metal, since MgO is not a gas.
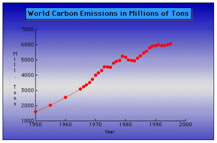
Carbon Dioxide: acidification of the oceans and the fertilization effect
The Hidden Effects of Global Warming
By: Christopher Cassetta
Carbon Fertilization Effect: Carbon Dioxide Formation by Joseph Fowler III
Carbon Dioxide and the Earth by Shuvai Mtangi
Tyrie Johnson views the triclinic structure of Copper II Sulfate and the tetragonal structure of Calcium Sulfate.
Hayden Wihjelm views the cubic structure of table salt.
Illegal Hemp Production by Rachel Wiley
Hemp and Society by Kolton Hill
Victoria Boller holding the limiting reactant demo.
Limiting Reactants determine how much product that can be produced in a given reaction. Students learn that they will experience limiting reactants as a manager of a company. Managers take risks purchasing items to be sold at a higher cost, thus the number of items on hand is called the limiting reactants. Steve holds a flask that has 10 cm of magnesium metal and 50 ml of hydrochloric acid. Victoria holds a flask with 3 cm of magnesium metal and 50 ml of HCl. The reaction produces hydrogen gas which fills the balloon. The size of the balloon is determined by the amount of limiting reactant present.
Stoichiometry concerns predicting a theoretical amount of product before the reaction occurs, so that when the actual value is produced in the experiment a percent yield can be calculated. Students learned that when managers of clothing stores like Von Maur order goods they have a theoretical dollar amount expected from the sale. However, changes in the weather or a bad economy can cause the store to discount and the actual money generated from the sale is compared to the theoretical in order to determine if the product should be ordered again. Students allowed the product to dry over night and will calculate the actual copper produced from the reaction to determine the percent yield.
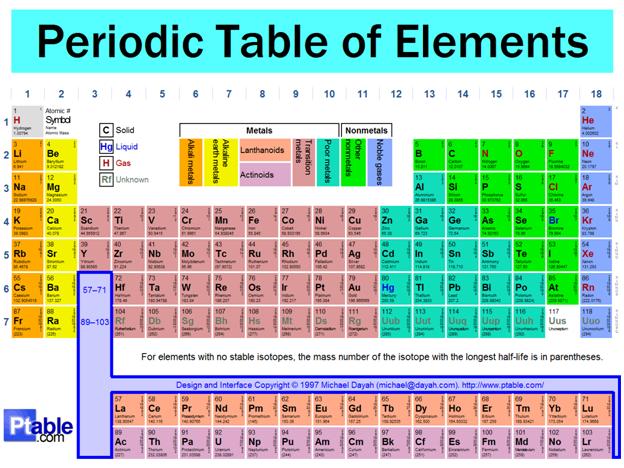
One of the most impressive and significant features of the Periodic Table of Elements is the appearance of vertical groups of elements, which form "families". Elements that are in the same "family" or "group" form compounds that are similar in formula and physical properties.
Zachary Minor speaks about the mole.
Group mole project
Jeremy Smith lectures on the mole.
Kevoia Boller makes the mole musical.
Obama discovers a mole in the White House. by Janar Abdulkathem and Krupaben Parmar
The Hyman Fire Piston demonstrates the rise in temperature when a fixed mass of gas is compressed. A portion of tinder supported inside a transparent cylinder is ignited when the contained air is compressed by a hand operated piston.
Dakota Chappell uses the combined law apparatus to show that when the volume is decreased the pressure increases and the temperature increases.
The combined gas law is a gas law that combines Charles's law, Boyle's law, and Gay-Lussac's law. There is no official founder for this law; it is merely an amalgamation of the three previously discovered laws. The combined gas law can be used to explain the mechanics where pressure, temperature, and volume are affected. For example: air conditioners, refrigerators and the formation of clouds, and also use in fluid mechanics and thermodynamics.
Final Exam Spring 2014
Click to take the Spring 2014 Final.
Rachel Wiley lite's a ritz peanut butter cracker.
Olivia uses her math skills to calculate calorie content in the food she tested.


Students use pocket book to solve Boyle's law problems
Density Column
How does the density column work?

Holli Grubs measures the volume of a liquid.

Density Cylinders
Use WordPress? The free EasyRotator for WordPress plugin lets you create beautiful WordPress sliders in seconds.
OK
Chemical Reactions
Use WordPress? The free EasyRotator for WordPress plugin lets you create beautiful WordPress sliders in seconds.
OK
Copper Chloride reacts with Aluminum
Use WordPress? The free EasyRotator for WordPress plugin lets you create beautiful WordPress sliders in seconds.
OK
Single replacement reaction
Sean demonstrates half life of an atom using pennies. Pennies have a 50% chance of heads or tails. Atoms have a 50% chance of changing into a new substance during their half life period.
Eddie with assistance from Trey counting nuclear change.
Cheyenne counts radioactive decay using pennies.
Use WordPress? The free EasyRotator for WordPress plugin lets you create beautiful WordPress sliders in seconds.
OK
Quantitative Analysis
The single replacement of copper II chloride with iron to form copper is an example of quantitative data. Students were evaluated based on the amount of copper produced.

Do not look directly at the burning magnesium.

Kindsey Gibson displays her happiness with observing magnesium burn.

The crucible can get very hot. Students are warned not to touch the white even though it may appear cold.

Cheyenne and Shawnice examine the spectrum of oxygen at 687nm and hydrogen at 656nm, 488nm, and 434nm.

Olivia and Gabby examine neon with the spectroscope.

Gun powder consists of 7.5 parts Carbon, 1.5 parts Potassium Nitrate, and 1 part Sulfur.

Katie C. burns metal to produce a visible line spectrum.
Acidification of the Ocean by Benjamin Christian Bird
Expository writing prompt on Hemp
Use WordPress? The free EasyRotator for WordPress plugin lets you create beautiful WordPress sliders in seconds.
OK
When compounds bond together they form crystal structures. Ice crystals form a Hexagonal structure.

Larry Fortney has the mono-clinic crystal structure of sucrose or table sugar in his mind by viewing it through a microscope.

Kendrick Burnett shows his expertise with balancing equations.

Iron and Sulfur are combined in a synthesis reaction with the addition of heat as a catalyst.

Malik Petty demonstrates the single replacement reaction of hydro chloric acid with zinc to produce hydrogen gas and zinc chloride. The hydrogen is lit with a wood split producing a pop.

Demi Gardner and Sean Brown observe the decomposition of calcium carbonate with the addition of heat.
Hemp Expository Paper by Stevie Kute
 Declaration of Independence was written on Hemp by Ryan Hay
Declaration of Independence was written on Hemp by Ryan Hay
-

 Determination of Chemical Formulas: Lab-AIDS
Determination of Chemical Formulas: Lab-AIDS
-


-


-


-


-


-


Use WordPress? The free EasyRotator for WordPress plugin lets you create beautiful WordPress sliders in seconds.
OK

Briana Turner records the procedure followed in the lab.

Marie Teria stirs the solution to increase the rate of reaction.

Katelynn Creak makes copper.

Copper filtered, so that it can dry and the actual yield determined.

Markeia Porter tries to determine the unknown formula.
Ben Bird demonstrates his creative song writing abilities.
Amazing mole by Dakota Chappell and Adam C.
Demi Gardner lectures to a group of friends about the mole.
Chris B asked for a mole of water, 18ml, or a sip of water.
Sarah Parker and Olivia Guerrero present the mole.

Victoria Kilpatrick demonstrates a change in temperature will cause a change in pressure. Gay-Lussac gas law

Boyles Law apparatus

Alston Sickles makes a measurement of volume change.

Shuvai Mtangi makes a measurement of volume change.
Test over Gas laws, click to take the test.

Students used a paper clip to hold the food item while it burned and melted the ice in the metal container.

Hayden Wihjelm burns an almond.

Cori Venning and Natasha Fenwick follow directions.
Calorimetry is a technique that is used to determine the heat involved in a chemical reaction. When determining the heat of combusiton of a substance or the caloric value of foods, the measurements are often made using a bomb calorimeter. In this experiement the student used a simplified set up measuring the volume of water that melted from the ice in the metal container. The heat of fusion for water is 80 cal/g, so this value can be use to determine the energy content of food.

Are you true blue?

Justin Zaring Conservation of Mass 1

Justin Zaring Conservation of Mass 2
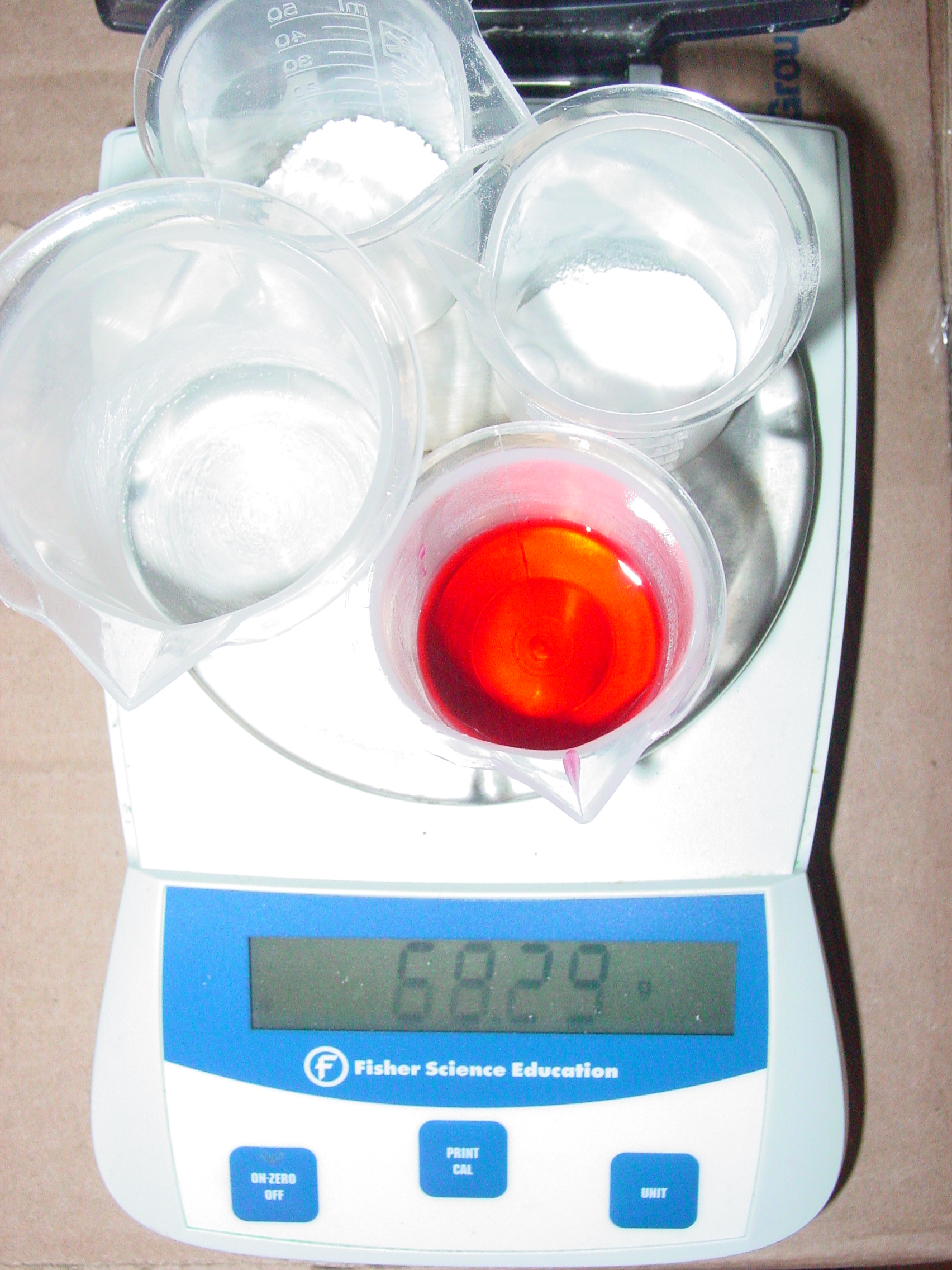

Was conservation of mass observed? Explain.
-


-


-


-


-


-


-


This jQuery slider was created with the free EasyRotator software from DWUser.com.
Use WordPress? The free EasyRotator for WordPress plugin lets you create beautiful WordPress sliders in seconds.
OK
Chromatography - separation of a mixture
-


-


-


-


This jQuery slider was created with the free EasyRotator software from DWUser.com.
Use WordPress? The free EasyRotator for WordPress plugin lets you create beautiful WordPress sliders in seconds.
OK
Forensic scientists use certain chromatography techniques to analyze drugs and dyes in cloth fibers.
-


-


-


-


-


This jQuery slider was created with the free EasyRotator software from DWUser.com.
Use WordPress? The free EasyRotator for WordPress plugin lets you create beautiful WordPress sliders in seconds.
OK
Calculate the Rf value for each color by measuring the distance the substance travels divided by the distance developer traveled.

Slime
Elmer's Glue is made up of polyvinyl acetate, which reacts with water to some extent to replace some of
the acetate groups with OH (alcohol) groups. The B-OH groups on the borax molecules react with the
acetate groups on the glue molecules (relatively long polymer chains) to eliminate acetic acid and form new
bonds between the borax and two glue molecules. The linking of two glue molecules via one borax molecule
is called polymer cross-linking and it makes a bigger polymer molecule, which is now less liquid-like and
more solid.

Rachel Denny makes slime.

Jeremy Bonner examines his magnesium oxide ash. Christopher Cassetta Christopher Burton, and Zeb Fields measured out 0.5g Mg and cremated it in a crucible to obtain 0.75g MgO. The 0.5g Mg is mathematically calculated to produce 0.84g MgO. Thus the 0.75/0.84 g MgO x 100 produced an 89% yield.
-


-


-


-


-


-


-


-


-


-


-


-


This jQuery slider was created with the free EasyRotator software from DWUser.com.
Use WordPress? The free EasyRotator for WordPress plugin lets you create beautiful WordPress sliders in seconds.
OK
Test over periodic trends, click here to take the test.

Sean Brown examines Neon gas through a project star spectrometer to determine the spectrum.
 Neon has an orange red color and Mercury has a light blue transmission.
Neon has an orange red color and Mercury has a light blue transmission.

Matthew F. burns some potassium chloride.

Kevin burns an unknown metal. Part of the lab experiment concerned the identification of an unknown from the testing of known's.
Carbon Dioxide Emmisons: by Jenny Daily
Carbon Dioxide : How much is too much? by Sydney White

Anion notes showing steps for testing four known ions for determination of an unknown.
-


-


-


-


-


-


-


-


-


-


-


-


-


This jQuery slider was created with the free EasyRotator software from DWUser.com.
Use WordPress? The free EasyRotator for WordPress plugin lets you create beautiful WordPress sliders in seconds.
OK
Detectives in mystery novels often rush evidence from the crime scene to the lab for analysis. In this experiment, the student became the chemical detective. They conducted laboratory analysis to determine the ionic composition of an unknown anion solution. The four known anions were as follows: Chloride, Phosphate, Sulfate, and Carbonate. The teacher made the unknown solution to be Sulfate. The students performed the required tests and most got the identification of the unknown correct.

Steps for testing known cat ions for the determination of an unknown.
-


-


-


-


-


-


-


-


-


-


-


-


-


-


-


-


This jQuery slider was created with the free EasyRotator software from DWUser.com.
Use WordPress? The free EasyRotator for WordPress plugin lets you create beautiful WordPress sliders in seconds.
OK
A cat ion is an ion that has a positive charge. It has a positive charge, because the atom has lost an electron and now the atom is not balanced or neutral. The electron is negative and the proton is positive. So when an electron leaves the atom the proton is the unit that provides the positive charge to the ion. Students use caution with Sulfuric Acid, Hydrochloric Acid, Sodium Hydroxide, and Potassium Chicano.
Laughing Grass and the Law by Zachary Stevenson
 You tube project rubric
You tube project rubric
 Hemp in America by Sarah Parker
Hemp in America by Sarah Parker
-


-


-


-


-


-


-


-


-


-


-


This jQuery slider was created with the free EasyRotator software from DWUser.com.
Use WordPress? The free EasyRotator for WordPress plugin lets you create beautiful WordPress sliders in seconds.
OK
Making Table Salt
-


-


-


-


-


-


-


-


-


-


-


-


-


-


-


-


This jQuery slider was created with the free EasyRotator software from DWUser.com.
Use WordPress? The free EasyRotator for WordPress plugin lets you create beautiful WordPress sliders in seconds.
OK
Best method for making NaCl, since there is usually more than one way to solve a problem. Students make the same product using different reactants and determine which method has the higher percent yield.

Copper forming from the single replacement reaction of copper II Sulfate with steel wool.

Jacob Baldwin preparing the funnel to filter the copper.

Christopher Cassetta reads and follows the directions.
-


-


-


-


-


-


-


-


-


This jQuery slider was created with the free EasyRotator software from DWUser.com.
Use WordPress? The free EasyRotator for WordPress plugin lets you create beautiful WordPress sliders in seconds.
OK
In this experiment students determined the elements that are in the same family and the formulas of the compounds formed by observing the properties of compounds of the elements.

Lakale M uses the franklin apparatus to show how stressed she is after taking the science diagnostic test for the course.

The Temperature-Pressure Law was studied by another French scientist. Joseph Gay-Lussac studied hot-air balloons in the early 1800’s. He studied the relationship between Temperature and Pressure. He found that when the temperature is increased, the Pressure of a gas also increased, and vice versa. He found the relationship was linear, but not quite direct. Similar to Charles’ Law, we must use The Kelvin temperature scale in order for the relationship to be direct. All we have to do is add 273 to our temperature celcius, and we get a direct relationship between volume and temperature.

Demi Gardner demonstrates the franklin apparatus.

Sophia Musa with the palm glass demonstrates the contrasting effects of heat and gravity on a volatile liquid.
The glass is filled with a colored liquid, such as methylene chloride, having a low vapor point. The air in the tube ahs been partially evacuated. The apparatus is held by hand at an angle, heat from the hand providing the input of energy.
A fluid with a low vapor point will evaporate with a relatively low input of thermal energy. The lowered pressure due to the evacuation of the tube enables the fluid to evaporate even more easily with the thermal input from the student’s hand.
Once the fluid reaches the upper bulb, it will cool and return to a liquid state. As it flows down to the hand-held bulb under the influence of gravity, it is reheated and evaporates again.

Carson Mesmer makes a measurement of volume change.

Gabby Wihjelm and Marie Teria burn goldfish crackers to determine calorie content.

Mike Hamm and Ebony Harl observe the heat given off by a pecan.

Isaiah McMillen and Tyrie Johnson burn breakfast cereal.

Malik Petty is always on task and doing what he is suposed to do. He is a great student.
All Content © copyright 2000-2014 All rights reserved.

Are you true blue?

Justin Zaring Conservation of Mass 1

Justin Zaring Conservation of Mass 2


Was conservation of mass observed? Explain.
Use WordPress? The free EasyRotator for WordPress plugin lets you create beautiful WordPress sliders in seconds.
OK
Chromatography - separation of a mixture
Use WordPress? The free EasyRotator for WordPress plugin lets you create beautiful WordPress sliders in seconds.
OK
Forensic scientists use certain chromatography techniques to analyze drugs and dyes in cloth fibers.
Use WordPress? The free EasyRotator for WordPress plugin lets you create beautiful WordPress sliders in seconds.
OK
Calculate the Rf value for each color by measuring the distance the substance travels divided by the distance developer traveled.
Slime
Elmer's Glue is made up of polyvinyl acetate, which reacts with water to some extent to replace some of
the acetate groups with OH (alcohol) groups. The B-OH groups on the borax molecules react with the
acetate groups on the glue molecules (relatively long polymer chains) to eliminate acetic acid and form new
bonds between the borax and two glue molecules. The linking of two glue molecules via one borax molecule
is called polymer cross-linking and it makes a bigger polymer molecule, which is now less liquid-like and
more solid.
Rachel Denny makes slime.

Jeremy Bonner examines his magnesium oxide ash. Christopher Cassetta Christopher Burton, and Zeb Fields measured out 0.5g Mg and cremated it in a crucible to obtain 0.75g MgO. The 0.5g Mg is mathematically calculated to produce 0.84g MgO. Thus the 0.75/0.84 g MgO x 100 produced an 89% yield.
Use WordPress? The free EasyRotator for WordPress plugin lets you create beautiful WordPress sliders in seconds.
OK
Test over periodic trends, click here to take the test.

Sean Brown examines Neon gas through a project star spectrometer to determine the spectrum.
 Neon has an orange red color and Mercury has a light blue transmission.
Neon has an orange red color and Mercury has a light blue transmission.

Matthew F. burns some potassium chloride.

Kevin burns an unknown metal. Part of the lab experiment concerned the identification of an unknown from the testing of known's.
Carbon Dioxide Emmisons: by Jenny Daily
Carbon Dioxide : How much is too much? by Sydney White

Anion notes showing steps for testing four known ions for determination of an unknown.
Use WordPress? The free EasyRotator for WordPress plugin lets you create beautiful WordPress sliders in seconds.
OK
Detectives in mystery novels often rush evidence from the crime scene to the lab for analysis. In this experiment, the student became the chemical detective. They conducted laboratory analysis to determine the ionic composition of an unknown anion solution. The four known anions were as follows: Chloride, Phosphate, Sulfate, and Carbonate. The teacher made the unknown solution to be Sulfate. The students performed the required tests and most got the identification of the unknown correct.

Steps for testing known cat ions for the determination of an unknown.
Use WordPress? The free EasyRotator for WordPress plugin lets you create beautiful WordPress sliders in seconds.
OK
A cat ion is an ion that has a positive charge. It has a positive charge, because the atom has lost an electron and now the atom is not balanced or neutral. The electron is negative and the proton is positive. So when an electron leaves the atom the proton is the unit that provides the positive charge to the ion. Students use caution with Sulfuric Acid, Hydrochloric Acid, Sodium Hydroxide, and Potassium Chicano.
Laughing Grass and the Law by Zachary Stevenson
 You tube project rubric
You tube project rubric
 Hemp in America by Sarah Parker
Hemp in America by Sarah Parker
Use WordPress? The free EasyRotator for WordPress plugin lets you create beautiful WordPress sliders in seconds.
OK
Use WordPress? The free EasyRotator for WordPress plugin lets you create beautiful WordPress sliders in seconds.
OK
Best method for making NaCl, since there is usually more than one way to solve a problem. Students make the same product using different reactants and determine which method has the higher percent yield.
Copper forming from the single replacement reaction of copper II Sulfate with steel wool.

Jacob Baldwin preparing the funnel to filter the copper.

Christopher Cassetta reads and follows the directions.
Use WordPress? The free EasyRotator for WordPress plugin lets you create beautiful WordPress sliders in seconds.
OK
In this experiment students determined the elements that are in the same family and the formulas of the compounds formed by observing the properties of compounds of the elements.

Lakale M uses the franklin apparatus to show how stressed she is after taking the science diagnostic test for the course.

The Temperature-Pressure Law was studied by another French scientist. Joseph Gay-Lussac studied hot-air balloons in the early 1800’s. He studied the relationship between Temperature and Pressure. He found that when the temperature is increased, the Pressure of a gas also increased, and vice versa. He found the relationship was linear, but not quite direct. Similar to Charles’ Law, we must use The Kelvin temperature scale in order for the relationship to be direct. All we have to do is add 273 to our temperature celcius, and we get a direct relationship between volume and temperature.

Demi Gardner demonstrates the franklin apparatus.

Sophia Musa with the palm glass demonstrates the contrasting effects of heat and gravity on a volatile liquid.
The glass is filled with a colored liquid, such as methylene chloride, having a low vapor point. The air in the tube ahs been partially evacuated. The apparatus is held by hand at an angle, heat from the hand providing the input of energy.
A fluid with a low vapor point will evaporate with a relatively low input of thermal energy. The lowered pressure due to the evacuation of the tube enables the fluid to evaporate even more easily with the thermal input from the student’s hand.
Once the fluid reaches the upper bulb, it will cool and return to a liquid state. As it flows down to the hand-held bulb under the influence of gravity, it is reheated and evaporates again.
Carson Mesmer makes a measurement of volume change.

Gabby Wihjelm and Marie Teria burn goldfish crackers to determine calorie content.

Mike Hamm and Ebony Harl observe the heat given off by a pecan.

Isaiah McMillen and Tyrie Johnson burn breakfast cereal.

Malik Petty is always on task and doing what he is suposed to do. He is a great student.
All Content © copyright 2000-2014 All rights reserved.





























 by Zhanee Masden
by Zhanee Masden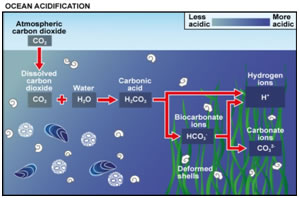

























.jpg)





.jpeg)