
Home | About Us | JCPS Home | EHS Science Video
- Chemistry Topics: 1) Matter and Measurement, 2) Atoms, Molecules, and Ions, 3) Stoichiometry, 4) Aqueous Solutions, 5) Thermochemistry, 6) Periodic Properties, 7) Solids, Liquids, and Gases, 8) Chemical Bonding, 9) Molecular Geometry, 10) Properties of Solutions, 11) Chemical Kinetics, 12) Chemical Equilibrium, 13) Acid-Base Chemistry, 14) Thermodynamics, 15) Electrochemistry, 16) Nuclear Chemistry
Xenon

by Austin Hegstad
Xenon was discovered on July 12th, 1898, by William Ramsay, and Morris Travers. While in London, England, These two chemists stumbled upon xenon after fractionally distilling liquid air and finding it in the deposit remaining. The name xenon comes from the Greek word ‘xenos’, meaning stranger. The chemical was probably named after this word because it was first mistaken to be a form of pure krypton.
Xenon is a colorless gas, but it emits a band of lines that cross the visual spectrum, the most powerful lines occur in the region of blue light, giving it a blue tint when charged with electricity. It can also give off a lavender tint. It has a density of 3.52g. It melts at  approximately -169.2°F. Xenon’s atomic radius is 108 pm. It has a specific heat of 20° C. Xenon is one of the heaviest gasses, and because of its low abundance, which is only one part of 64 million of the total mass of the solar system, it is more expensive than gasses that are lighter. It is a noble gas, which is one of five others.
approximately -169.2°F. Xenon’s atomic radius is 108 pm. It has a specific heat of 20° C. Xenon is one of the heaviest gasses, and because of its low abundance, which is only one part of 64 million of the total mass of the solar system, it is more expensive than gasses that are lighter. It is a noble gas, which is one of five others.
Xenon placed in a high voltage electric field.
 The electro negativity of xenon is 2.6(Pauling scale). Since it is a noble gas, it is inert to most common chemical reactions, meaning it will not react. Its first ionization energy is 1170.4 kJ. The second is 2046.4, and the third energy, 3099.4.
The electro negativity of xenon is 2.6(Pauling scale). Since it is a noble gas, it is inert to most common chemical reactions, meaning it will not react. Its first ionization energy is 1170.4 kJ. The second is 2046.4, and the third energy, 3099.4.
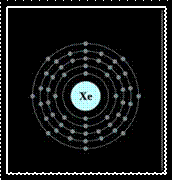
The electron configuration of an xenon atom.

Xenon is located in the p-block on the periodic table, in the 5th row. It is a part of the noble gas group.


To the left is a Bohr model of xenon, and to the right is the lewis dot structure.
Works Cited
Helmenstine, Anne Marie. "Xenon Facts - Periodic Table of the Elements." About.com Chemistry - Chemistry Projects, Homework Helm, Periodic Table. N.p., n.d. Web. 12 Oct. 2012. <http://chemistry.about.com/od/elementfacts/a/xenon.htm>.
"Xenon." Periodic Table of Elements and Chemistry. David D. Hsu, n.d. Web. 12 Oct. 2012. <http://www.chemicool.com/elements/xenon.html>.
MLA formatting by BibMe.org.