
Home | About Us | JCPS Home | EHS Science Video
- Chemistry Topics: 1) Matter and Measurement, 2) Atoms, Molecules, and Ions, 3) Stoichiometry, 4) Aqueous Solutions, 5) Thermochemistry, 6) Periodic Properties, 7) Solids, Liquids, and Gases, 8) Chemical Bonding, 9) Molecular Geometry, 10) Properties of Solutions, 11) Chemical Kinetics, 12) Chemical Equilibrium, 13) Acid-Base Chemistry, 14) Thermodynamics, 15) Electrochemistry, 16) Nuclear Chemistry
Technetium |
|
by Bradley Luttrell |
10/27/2012 |
|
In the early 1870’s Dmitri Mendeleev, after proposing many versions on the periodic table of elements was on the search for element 43. During the search Dmitri proposed that missing element 43 would go below manganese thus having similar properties. Throughout the late 1800s scientists were eager to discover missing element 43 but, the element would not be discovered until 1936. During an experiment at the University of Palermo in Sicily it was discovered. It was discovered by Carlo Perrier, during a visit to the United States were he persuaded Ernest Lawrence to let him take back some discarded cyclotron parts that had become radioactive. Perrier and his colleague attempted to prove through comparative chemistry that molybdenum activity was indeed element 43.Technetium became the first artificial element ever produced in the history of science. There is zero percent of element 43 found in nature because it is a man made element. On the periodic table of elements it can be classified as manmade and transition metal (Found in group 7, period 5).
Since technetium is actually forms of radioactive molybdenum all forms of it are radioactive and unstable. Technetium at a solid state is a grey metallic color, when solid the density is 11,500 kg m-3. Technetium has atomic radii of 1.82, when melted at twenty two hundred degrees Celsius it releases oxygen heptoxide creating a lot of heat. It is the lightest element on earth without any staple isotopes making all forms radioactive. Since it is a transition metal it is very difficult to find the valence electrons however we believe since it is in group 7 we assume it has seven valence electrons.
Electro negativity was developed by Linus Pauling, and on his scale he concluded that element 43 has an electro negativity of 1.9 Pauling units. Since the electro negativity is high it has a good chance of bonding with other elements similar to itself. The most useful isotope of technetium is technetium 99 m because of its short half life and its ability to bind to many biologically active molecules. When combined with tin it serves as a catalyst and it has the ability to attract red blood cells. Since we assume it has seven valence electrons it has a relatively high attraction to other elements.
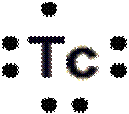 Lewis dot structure of Technetium
Lewis dot structure of Technetium
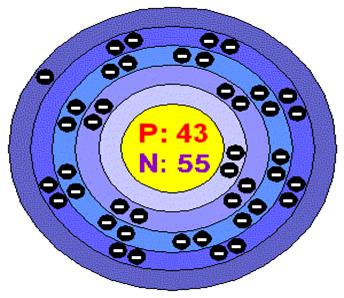 Bohrs model of technetium
Bohrs model of technetium
Electron configuration: 2,8,18,14,1
 My family and I with Tc
My family and I with Tc
http://www.bibme.org
http://en.wikipedia.org/wiki/Technetium
http://www.webelements.com/technetium/
http://www.pixlr.com
Works Cited:
Peacock, R. D.. The chemistry of technetium and rhenium,. Amsterdam: Elsevier Pub. Co., 1966. Print.
Winter, Mark. "WebElements Periodic Table of the Elements | Technetium | Essential information." Periodic Table of the Elements by WebElements. N.p., n.d. Web. 27 Oct. 2012. <http://www.webelements.com/technetium/>. MLA formatting by BibMe.org.
Winter, Mark. "WebElements Periodic Table of the Elements | Technetium | Essential information." Periodic Table of the Elements by WebElements. N.p., n.d. Web. 27 Oct. 2012. <http://www.webelements.com/technetium/>. MLA formatting by BibMe.org.