
Home | About Us | JCPS Home | EHS Science Video
- Chemistry Topics: 1) Matter and Measurement, 2) Atoms, Molecules, and Ions, 3) Stoichiometry, 4) Aqueous Solutions, 5) Thermochemistry, 6) Periodic Properties, 7) Solids, Liquids, and Gases, 8) Chemical Bonding, 9) Molecular Geometry, 10) Properties of Solutions, 11) Chemical Kinetics, 12) Chemical Equilibrium, 13) Acid-Base Chemistry, 14) Thermodynamics, 15) Electrochemistry, 16) Nuclear Chemistry
What is sulfer?

By: Trey Moses
History
Sulfer was discovered by the chinese before 2000 bc. The Chinese figured out it could be extracted from pyrite. A Song dynasty military treatise of 1044 AD described different formulas for Chinese black powder, which is a mixture of potassium nicrate (KNO3) , charcole and sulfur. According to the Ebers Papyrus, a sulfur ointment was use in ancient Egypt to treat granular eyelids.. The english translation of the bible commonly referred to burning sulfur as "brimstone", giving rise to the name of 'fire-and-brimstone' sermons, in which listeners are reminded of the fate of eternal damnation that await the unbelieving and unrepentant. It is from this part of the Bible that Hell is implied to "smell of sulfur".
Common uses of sulfer
- Fertilizer
- Wine
- Epsom salt
- Absorbed by plants
- Energy for bacteria

Physical properties
Occurs in big yellow crystals or masses. It is tasteless, odorless, and insoluble in water. It is a solid, and its density is alpha 2.07 g·cm−3. Its melting point is 239 degrees Fahrenheit. The atomic radius is 100 pm. The specific heat is .17. Large amounts of sulfuric acid, nearly 40 million tons, are used each year to make fertilizers, lead-acid batteries, and in many industrial processes. Smaller amounts of sulfur are used to vulcanize natural rubbers.
Chemical properties
The ionization energy of sulfer is 10.360 eV. The electro negativity is 2.58.

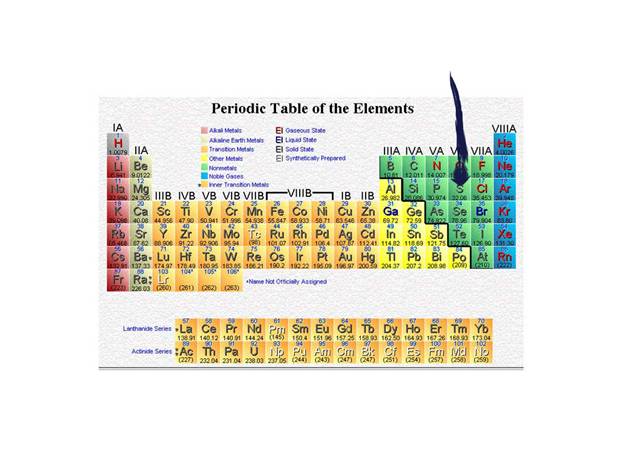
The arrow is pointed at sulfer.
- "Sulfur." Periodic Table of Elements and Chemistry. N.p., n.d. Web. 30 Oct. 2012. <http://www.chemicool.com/elements/sulfur.html>.
"Sulfur - Wikipedia, the free encyclopedia." Wikipedia, the free encyclopedia. N.p., n.d. Web. 30 Oct. 2012. <http://en.wikipedia.org/wiki/Sulfur#Characteristics>.
"Sulfur History." Georgia Gulf Sulfur Corporation. N.p., n.d. Web. 30 Oct. 2012. <http://www.georgiagulfsulfur.com/history
Solids - Specific Heats ." Engineering ToolBox . N.p., n.d. Web. 30 Oct. 2012. <http://www.engineeringtoolbox.com/specific-heat-solids-d_154.html>.
"It's Elemental - The Element Sulfur." Science Education at Jefferson Lab. N.p., n.d. Web. 30 Oct. 2012. <http://education.jlab.org/itselemental/ele016.html>.
"WebElements Periodic Table of the Elements | Sulfur | Electronegativity." Periodic Table of the Elements by WebElements. N.p., n.d. Web. 30 Oct. 2012. <http://www.webelements.com/sulfur/electronegativity.html>.
