
Home | About Us | JCPS Home | EHS Science Video
- Chemistry Topics: 1) Matter and Measurement, 2) Atoms, Molecules, and Ions, 3) Stoichiometry, 4) Aqueous Solutions, 5) Thermochemistry, 6) Periodic Properties, 7) Solids, Liquids, and Gases, 8) Chemical Bonding, 9) Molecular Geometry, 10) Properties of Solutions, 11) Chemical Kinetics, 12) Chemical Equilibrium, 13) Acid-Base Chemistry, 14) Thermodynamics, 15) Electrochemistry, 16) Nuclear Chemistry
Potassium (K) Element Writing Project
By Molly Rector
4th Period Chemistry, Mr. Steineker
This writing assignment addresses the element Potassium. It is a very interesting element due to the important role it plays in our daily lives. It is an essential mineral in our daily diet as well as its commercial and industrial uses ranging from fertilizer to its use in the production of soaps, bleach, and glass.
HISTORICAL BACKROUND:
- Potassium was invented in 1806 by Sir Humphry Davy in 1806. Davy electrolyzed dried potassium hydroxide which he had moistened by exposing it to the moist air in his laboratory.
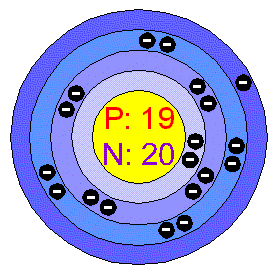
ELECTRON CONFIGURATION:
Potassium is a very important mineral for the proper function of all cells, tissues, and organs in our body. It is also an electrolyte, a substance that conducts electricity in the body, along with sodium, chloride, calcium, and magnesium. Potassium is crucial to the heart and plays a key role in skeletal and smooth muscle contraction, making it important for normal digestive and muscular function. Many foods contain potassium. Some examples include all meats, some types of fish and many fruits, vegetables, and legumes. Dairy products are also good sources of potassium.
PHYSICAL PROPERTIES:
Name: Potassium
Symbol: K
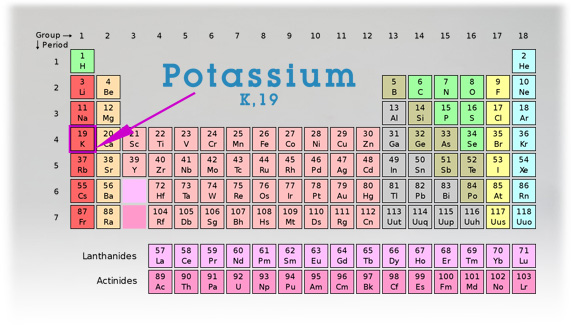
Atomic Number: 19
Atomic Mass: 39.0983 amu
Atomic Radius: 235
Abundance: 8th most abundant on earth.
Specific heat: 0.753
Boiling Point: 774.0 °C (1047.15 K, 1425.2 °F)
Number of Protons/Electrons: 19
Number of Neutrons: 20
Classification: Alkali Metal
Crystal Structure: Cubic
Melting Point: 63.65 °C (336.8 K, 146.57 °F)
Density: @ 293 K: 0.862 g/cm3
State: Solid
Appearance: Silvery
Potassium is the second least dense metal, after lithium. It is a soft solid that has a low melting point and can easily be cut. Freshly cut potassium is silvery in appearance, but it begins to tarnish toward a grayish color immediately after being exposed to the air.
CHEMICAL PROPERITES:
Electro negativity: 0.82
Ionization energy: 418.8
Reaction partners: Water
Elemental potassium does not occur in nature because it reacts violently with water. Potassium makes up about 2.6% of the weight of the Earth's crust and is the seventh most abundant element.
OBJECTS CONTAINING POTASSIUM:



PERIODIC TABLE:
LEWIS DOT STRUCTURE:
ELEMENT BLOCK:

BOHR MODEL:
Works Cited
Burkhardt, Elizabeth R., Norman N. Greenwood, Alan Earnshaw, Arnold F. Holleman, Egon Wiberg, Niles Wiberg, and H. Schultz. "Potassium - Wikipedia, the free encyclopedia." Wikipedia, the free encyclopedia. N.p., n.d. Web. 31 Oct. 2012. <http://en.wikipedia.org/wiki/Potassium>. Burkhardt, Elizabeth R. et al. (2006). "Potassium and Potassium Alloys". Ullmann's Encyclopedia of Industrial Chemistry. A22. pp. 31–38. doi:10.1002/14356007.a22_031.pub2. ISBN 3-527-30673-0. Greenwood, Norman N; Earnshaw, Alan (1997). Chemistry of the Elements (2 ed.). Oxford: Butterworth-Heinemann. ISBN 0-08-037941-9. Holleman, Arnold F.; Wiberg, Egon; Wiberg, Nils (1985). "Potassium" (in German). Lehrbuch der Anorganischen Chemie (91–100 ed.). Walter de Gruyter. ISBN 3-11-007511-3. Schultz, H. et al. (2006). "Potassium compounds". Ullmann's Encyclopedia of Industrial Chemistry. A22. pp. 39–103. doi:10.1002/14356007.a22_031.pub2. ISBN 3-527-30673-0.
Herbert, Victor , and Jenell J. Subak-Sharp. "Vitamins and Minerals." The Mount Sinai School of Medicine Complete Book of Nutrition. New York: St. Martin's Press, 1990. 89 - 111. Print.
"Potassium." University of Maryland Medical Center | Home. University of Maryland Medical System, n.d. Web. 31 Oct. 2012. <http://www.umm.edu/altmed/articles/potassium-000320.htm>. © 2011 University of Maryland Medical Center (UMMC). All rights reserved. UMMC is a member of the University of Maryland Medical System, 22 S. Greene Street, Baltimore, MD 21201. TDD: 1-800-735-2258 or 1.866.408.6885http://www.umm.edu/altmed/articles/potassium-000320.htm#ixzz2AwA2paXs
Van Vlack, Lawrence H.. Elements of Material Science. Third Edition ed. Reading, Massachusetts: Addison-Wesley Publishing Company, 1975. Print.
MLA formatting by BibMe.org.

