
Home | About Us | JCPS Home | EHS Science Video
- Chemistry Topics: 1) Matter and Measurement, 2) Atoms, Molecules, and Ions, 3) Stoichiometry, 4) Aqueous Solutions, 5) Thermochemistry, 6) Periodic Properties, 7) Solids, Liquids, and Gases, 8) Chemical Bonding, 9) Molecular Geometry, 10) Properties of Solutions, 11) Chemical Kinetics, 12) Chemical Equilibrium, 13) Acid-Base Chemistry, 14) Thermodynamics, 15) Electrochemistry, 16) Nuclear Chemistry
By Aundreya Chandler



Platinum was discovered by South American peoples who produced artifacts of a white gold-platinum alloy. For the Spanish Conquistadors of the 16th century, platinum was a nuisance. While panning for gold in New Granada they were puzzled by some white metal nuggets which were mixed with the nuggets of gold and which were difficult to separate. The Spanish called this metal Platina, a diminutive of Plata, the Spanish word for silver. Some thought that the platinum was a sort of unripe gold, so that for many years it had no value except as a means of counterfeiting.
The first written account of platinum was from Julius C Scaliger in 1557. He describes it as a strange metal found in mines between Panama and Mexico and wrote that no fire or any of the Spanish arts could melt it.
In 1783 French chemist Francois Chabaneaus discovered and patented a method of producing workable platinum. However the quality of the metal was still very inconsistent from batch to batch – unknown to him, there were impurities of, as then, undiscovered metals.
English chemist William H Wollaston developed a commercial process for producing pure platinum in the early 19th century. In the course of his studies on platinum ores he also discovered the metals osmium, iridium, rhodium and palladium – the elements which had made Chabaneaus’ work so frustrating.
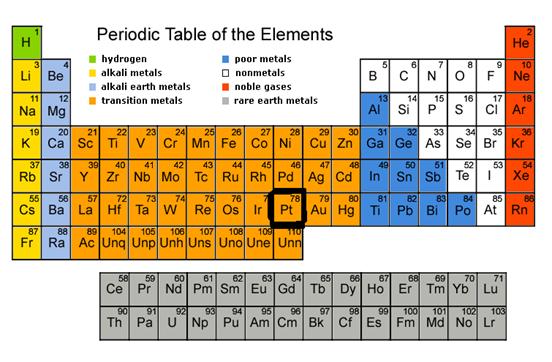
Antonio de Uolla was credited with the discovery of Platinum. He was born on January 12, 1716, Seville and died on July 3, 1795, Isla de León. The element name comes from the Spanish word ‘platina’ meaning little silver.

Uolla was credited with the discovery of Platinum.

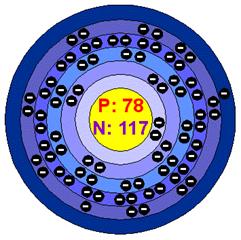
Platinum is a precious metal; soft, silvery-white, and dense with a beautiful lustrous sheen. It is malleable and ductile and has a high melting point. Platinum does not oxidize in air even at high temperatures and is unaffected by common acids. It dissolves in aqua regia (mixture of nitric acid and hydrochloric acid in the ratio 1:3) forming chloroplatinic acid (H2PtCl6 ). It is also corroded by halogens, cyanides, sulfur, and caustic alkalis. It is a solid state. The density of platinum is 21.4 g/cm3. The Atomic Volume is 9.1 cm3/mole. The Atomic Radius is 1.39 angstroms. The Covalent Radius is pm and it isn’t radioactive. The Melting Point is 1772.0 °C, Boiling Point is 3827.0 °C, The Heat of Fusion is 19.66 kJ/mole, and the Heat of Vaporization is 510.45 kJ/mole. The Oxidation States are +4,2, The Electrons Per Shell: 2 8 18 32 17 1, and the Electron Configuration is [Xe] 4f14 5d9 6s1 (1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d9 6s1), It was also discovered in the United Kingdom.


The electro negativity of platinum is 2.3 (Pauling Scale), the electron affinity is -205 kJ/mol, 1st Ionization Energy is 870 kJ/mol. The specific Heat Capacity is 0.13 J g−1 K−1, Thermal Conductivity is 71.6 W·m−1·K−1. Platinum does not normally react with air or water.
Careful control of the reaction between platinum metal and fluorine gas gives either the volatile platinum(VI) fluoride, PtF6 or the tetrameric platinum(V) fluoride, (PtF5)4. The latter product disproportionates into platinum(VI) fluoride and platinum(IV) fluoride, PtF4.
Pt(s) + 3F2(g) ![]() PtF6(s) [dark red]
PtF6(s) [dark red]
4Pt(s) + 10F2(g) ![]() (PtF5)4(s) [deep red]
(PtF5)4(s) [deep red]
(PtF5)4(s) ![]() PtF6(s) + PtF4(s) [yellow brown]
PtF6(s) + PtF4(s) [yellow brown]
PtCl4, PtBr4 and PtI4 are formed in the reactions of platinum metal and chlorine, Cl2, bromine, Br2, or iodine, I2.
Pt(s) + 2Cl2(g) ![]() PtCl4(s) [red brown]
PtCl4(s) [red brown]
Pt(s) + 2Br2(g) ![]() PtBr4(s) [brown black]
PtBr4(s) [brown black]
Pt(s) + 2I2(g) ![]() PtI4(s) [brown black]
PtI4(s) [brown black]
PtCl2 is also formed in the controlled reaction of platinum metal and chlorine. Depending upon the reaction conditio9ns, one of two different forms of PtCl2 is formed.
Pt(s) + Cl2(g) ![]() PtCl2(s) [dark red or olive green]
PtCl2(s) [dark red or olive green]


Platinum is a Transition Metal. Platinum’s Lewis Dot Structure is also unknown.


Xe 4f14 5d9 6s1

According to the Centers for Disease Control and Prevention, short-term exposure to platinum salts may cause irritation of the eyes, nose, and throat, and long-term exposure may cause both respiratory and skin allergies. The current OSHA standard is 2 micrograms per cubic meter of air averaged over an 8-hour work shift. Platinum-based antineoplastic agents are used in chemotherapy, and show good activity against some tumors.
As platinum is a catalyst in the manufacture of the silicone rubber and gel components of several types of medical implants (breast implants, joint replacement prosthetics, artificial lumbar discs, vascular access ports, etc.), the possibility platinum could enter the body and cause adverse effects has merited study. The Food and Drug Administration and other institutions have reviewed the issue and found no evidence to suggest toxicity in vivo.


The image above is a virtual representation of platinum metal calculated by Patrick Callet using the complex diectric function of the element only.

1,000 cubic centimeters of 99.9% pure platinum, worth about US$970,600 at 14 July 2012 prices!

Platinum is the World’s Most Precious Metal and its prices can vary form $100 Thousand – Over $3 Million

http://www.chemicool.com/elements/platinum.html
http://www.chemicalelements.com/elements/pt.html
http://www.chemicalelements.com/elements/pt.html
http://www.jewelrycentral.com/categories.asp?cID=120
http://www.ultrawheel.com/wheel_brand.cfm?brand=2
http://www.britishmuseum.org/research/search_the_collection_database/search_object_details.aspx?objectid=1336355&partid=1&output=Terms%2F!!%2FOR%2F!!%2F18779%2F!%2
file:///E:/Platinum%20Project/Element%20Help.htm
http://en.wikipedia.org/wiki/Antonio_de_Ulloa
http://www.joseibi.com/precious-metals-in-skin-care-breakthrough-antiaging-solution-or-unsafe/
http://www.medsafe.govt.nz/hot/alerts/HerbalRemedies/HerbalRemediesPics1.asp
http://www.amazon.com/Contemporary-Diamond-containing-Scattered-Brilliant/dp/B0087HJ2VG
http://www.ehow.com/how_7537800_identify-hallmarks.html
http://www.tinyjewelbox.com/bridal/item_retail.asp?parentId=18&id=171&item=558
http://aceszone-shop.blogspot.com/
http://www.biteki.com/article/2011/08/29/22154/
http://www.platinum.matthey.com/news-archive/drop-in-ruthenium-sales-hits-brush-engineered-metals/
http://www.costco.ca/Round-Diamond-Stud-Earrings-(1.00-ctw)-Platinum.product.10395865.html