
Home | About Us | JCPS Home | EHS Science Video
- Chemistry Topics: 1) Matter and Measurement, 2) Atoms, Molecules, and Ions, 3) Stoichiometry, 4) Aqueous Solutions, 5) Thermochemistry, 6) Periodic Properties, 7) Solids, Liquids, and Gases, 8) Chemical Bonding, 9) Molecular Geometry, 10) Properties of Solutions, 11) Chemical Kinetics, 12) Chemical Equilibrium, 13) Acid-Base Chemistry, 14) Thermodynamics, 15) Electrochemistry, 16) Nuclear Chemistry
Oxygen
by Lauren Falk
1. Oxygen was discovered in 1772 for the first time by Swedish Chemist, Carl Wilhelm Scheele. Joseph Priestly, an English chemist, discovered oxygen in 1774 and published his findings the same year. Antoine Lavoisier, a French chemist, also discovered oxygen in 1775, was the first to recognize it as an element, and named it oxygen, which comes from a Greek word that means acid former. They all found it the same way though, through heating different elements along with red hot manganese oxide that formed a gas. They also discovered that oxygen came from plants. They found this out by burning a sprig of mint and enclosing it in a box, he watched it closely with a light and saw smoke. This is what they called oxygen.
2. Oxygen is colorless, odorless and tasteless diatomic gas. The boiling point is 183C °C (90 K) and the melting point is 218.4° C (-361° F). The relative density of oxygen to air is 1.1053. Oxygen is a gas that is slightly heavier then air. The abundance of oxygen is 8 and the atomic radius is nearly 0.265 x 10 -10 m. The specific heat of oxygen is 1.40 but it varies.
3. Chemical properties of oxygen are the electro negativity which is 3.4 and the ionization energy which is 1314 kJ/mol. The reaction partners for oxygen are hydrogen and fluorine.
4.


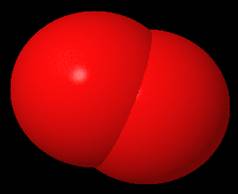
Oxygen
5. ¯
Group- chalcogen group
Period- 2
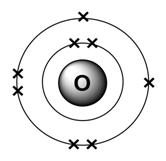
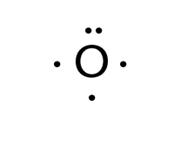
6. Electron configuration and Lewis Dot structure;:
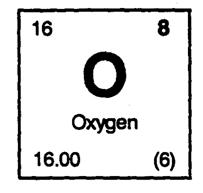
7. Element Block for oxygen:
9. Bohr Model for oxygen:
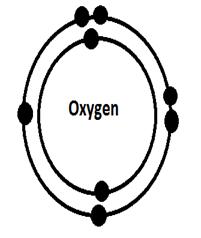
The nucleus is in the middle of the model and on the outside are the protons, neutrons and electrons.
7. Bibliography:
http://en.wikipedia.org/wiki/Oxygen
http://www.chemicool.com/elements/oxygen.html
http://sourcing.indiamart.com/engineering/articles/oxygen-gas-properties/
Periodic table of elements