
Home | About Us | JCPS Home | EHS Science Video
- Chemistry Topics: 1) Matter and Measurement, 2) Atoms, Molecules, and Ions, 3) Stoichiometry, 4) Aqueous Solutions, 5) Thermochemistry, 6) Periodic Properties, 7) Solids, Liquids, and Gases, 8) Chemical Bonding, 9) Molecular Geometry, 10) Properties of Solutions, 11) Chemical Kinetics, 12) Chemical Equilibrium, 13) Acid-Base Chemistry, 14) Thermodynamics, 15) Electrochemistry, 16) Nuclear Chemistry

Element Writing Project
November 2, 2012
Victoria Jarrett
Mr. Steineker
6th Period
Iron is an element that is considered to be a transition metal, which transitional metals are the elements that consists of Groups 3 through Groups 12 of the periodic table. Transitional metals are typical metals because they are shiny, bright, silvery solids that have higher melting points as well as they conduct electricity and heat well. This paper will discuss many fascinating facts about this element such as the historical background, physical properties and the chemical properties. In addition this paper will provide graphics of this element as well the Bohr model and the electron configuration and Lewis Dot Structure.
Historical Background
The discovery of iron dates back as far as prehistoric times, the beginning of human history (1200 B.C.) is known as the Iron Age, which is also the time that humans began and learned how to use iron metal. Iron is an element that does not happen to be a free element within the earth, humans could not use iron as a metal until it was freed from its compounds. Once this had occurred the element could be used as a metal that was useful for making tools, household implements, and weapons. An interesting fact of the historical background information of the element of iron is that there is a pillar made of this metal in Delhi, India that was built in 415 A.D. that still stands. The first known use of iron by humans came from meteorites.
Physical Properties

The physical property of the element of iron is that it is a white-silvery or even grayish metal. This metal is malleable and ductile, which means that it can be pounded into thin sheets or pulled into thin strands of wires. It is a tensile strength metal because it can be stretched without breaking. An important fact of the physical property of this element is that it is only one of the three elements that have a naturally occurring magnetism. The melting point of iron is 2,797 degrees Fahrenheit and the boiling point of iron is 5,400 degrees Fahrenheit.
Chemical Properties
The element of iron is an active metal, which it will readily combine with oxygen in the moist air. This reaction is known as iron oxide or otherwise known as rust. Another chemical reaction of this element occurs with hot water and steam which produces hydrogen gas. This element will dissolve in several acids and will react with many other elements on the periodic table.


Periodical Table, Electron Configuration, and Lewis Dot Structure
Iron is located on the periodical table within Group 8, which is part of the transitional metals. It has an atomic number of 26, which is why it is listed Fe 26 on the periodical table. It has 26 electrons and 26 protons and 30 neutrons. Iron has an atomic mass of 55.847 as well. The state of this element is a solid. The electron configuration of iron is [Ar] 3d6 4s2, the following picture is the Lewis Dot Structure of iron oxide or rust and the Bohr model of the element of Iron.
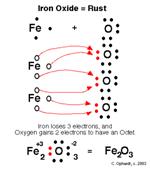
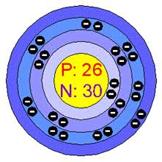
Conclusion
This paper has discussed many fascinating facts about the element of iron including the historical facts of how and when this element was discovered. This paper also discussed the physical and chemical properties of iron such as how iron, oxygen, and moist air creates iron oxidation. Another important fact that this paper has discussed was the information that describes this element on the periodical table.
References
"Iron." Chemicool Periodic Table. Chemicool.com. 06 Oct. 2012. Web. 10/30/2012
<http://www.chemicool.com/elements/iron.html>.
Helmenstine, Anne Marie. Iron Facts Chemical & Physical Properties of Iron. N.p., n.d. Web. 30 Oct. 2012. <http://chemistry.about.com>.
Hasan, Heather. Iron. First ed. New York: The Rosen Publishing Group, 2005. 6-16. Print.
Cooper, Sharon. The Periodic Table Mapping The Elements. Mankato: Compass Point Books, 2007. Print.