
Home | About Us | JCPS Home | EHS Science Video
- Chemistry Topics: 1) Matter and Measurement, 2) Atoms, Molecules, and Ions, 3) Stoichiometry, 4) Aqueous Solutions, 5) Thermochemistry, 6) Periodic Properties, 7) Solids, Liquids, and Gases, 8) Chemical Bonding, 9) Molecular Geometry, 10) Properties of Solutions, 11) Chemical Kinetics, 12) Chemical Equilibrium, 13) Acid-Base Chemistry, 14) Thermodynamics, 15) Electrochemistry, 16) Nuclear Chemistry
 Hydrogen
Hydrogen
Morgan Zachary
Historical Background- Henry Cavendish discovered hydrogen in 1766. It was discovered while experimenting with iron and acids in the United Kingdom.
Physical Properties |
Chemical Properties |
Colorless |
Reacts easily with other chemical substances |
Highly flammable |
Slightly more soluble in organic solvents |
Light in weight |
Does not usually react with other chemicals at room temperature |
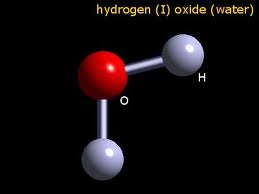
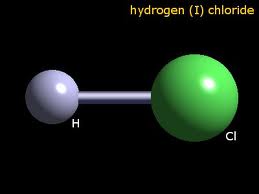 Photographs
Photographs

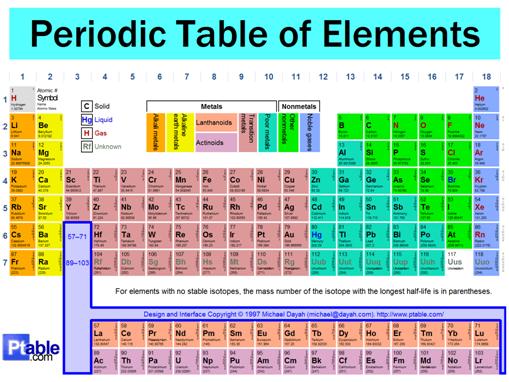
Location- It is located in group 1, s-block on the periodic table.
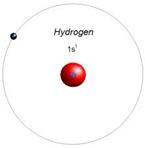
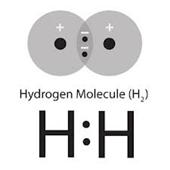
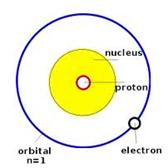
Lewis dot structure Electron Configuration Bohr model

Atomic Number: 1
Atomic Weight: 1.00794
Melting Point: 13.81 K (-259.34°C or -434.81°F)
Boiling Point: 20.28 K (-252.87°C or -423.17°F)
Density: 0.00008988 grams per cubic centimeter
Phase at Room Temperature: Gas
Element Classification: Non-metal
Period Number: 1 Group Number: 1 Group Name: none
Source: en.wikipedia.org/wiki/Hydrogen,
education.jlab.org/itselemental/ele001.
htmlwww.webelements.com/hydrogen/www.chemicalelements.com/elements/h.html
MLA formatting by BibMe.org.
