
Home | About Us | JCPS Home | EHS Science Video
- Chemistry Topics: 1) Matter and Measurement, 2) Atoms, Molecules, and Ions, 3) Stoichiometry, 4) Aqueous Solutions, 5) Thermochemistry, 6) Periodic Properties, 7) Solids, Liquids, and Gases, 8) Chemical Bonding, 9) Molecular Geometry, 10) Properties of Solutions, 11) Chemical Kinetics, 12) Chemical Equilibrium, 13) Acid-Base Chemistry, 14) Thermodynamics, 15) Electrochemistry, 16) Nuclear Chemistry
Element Helium

By Alina Levitt
Helium was first discovered as an unknown yellow line in sunlight during a solar eclipse in 1868 by Jules Janssen who was a French astronomer. However, he shares the credit with Norman Lockyer because he was the first to propose that the line Janssen discovered was in fact a new element. The formal discovery was then made official in 1895 by two Swedish chemists by the names Per Teodo Cleve and Nils Abraham Langlet, who found helium radiating from the uranium ore cleveite.
The physical properties of helium are that it is a very light, inert, colorless gas. Helium’s chemical properties make it the element that has the lowest melting point. It is the only liquid that cannot be solidified by lowering the temperature. It will remain a liquid down to absolute zero at ordinary pressures, but can be solidified by increasing the pressure. Helium is known as a inert or noble gas which means it is very un-reactive.



The electron configuration shown by the Lewis Dot Structure indicates that helium has only two electrons.
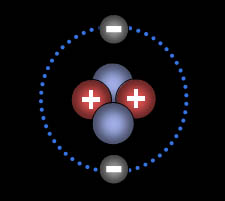
The Bohr Model will show you that helium is the only atom with a full shell of two electrons. -2e++2p=0 Net Charge.
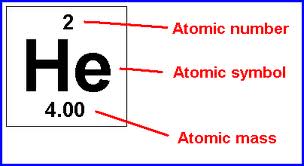
Helium Element Block
SOURCES:
www.wikipedia.com
www.chemistry.about.com
www.pixlr.com
www.google.com
www.chem1.com/acad/webtext/virtualtextbook.html
Basic Chemistry by Karen C. Timberlake
Introduction to Chemistry by David W. Bell