
Home | About Us | JCPS Home | EHS Science Video
- Chemistry Topics: 1) Matter and Measurement, 2) Atoms, Molecules, and Ions, 3) Stoichiometry, 4) Aqueous Solutions, 5) Thermochemistry, 6) Periodic Properties, 7) Solids, Liquids, and Gases, 8) Chemical Bonding, 9) Molecular Geometry, 10) Properties of Solutions, 11) Chemical Kinetics, 12) Chemical Equilibrium, 13) Acid-Base Chemistry, 14) Thermodynamics, 15) Electrochemistry, 16) Nuclear Chemistry
Gold Essay

By: Michael Grosse
Gold is a rare element that really can’t be traced, historians believe that the ancient Egyptians or Nubians were the first to find gold around 2600-3000 BC. As far as how the gold was found no one exactly knows, most historians believe that the first amounts of gold were found through agriculture in the Fertile Crescent region or present day Iraq.
Gold has a yellowish metallic appearance it has a density of 19.30 g/cm, it has a melting point of 1064.18 degrees Celsius, an atomic radius of 144 pm, it has a specific heat of 0.126 J/Gm, and has an abundance of a staggering 0.0005 parts per million. Basically this means that gold is very malleable and can be easily shaped, it is very rare, and most scientists classify it as a transition metal.
It’s chemical properties are quite impressive, it has an electronegativity rating of 2.54 on the Pauling scale. It’s ionization energies are as follows: 1st: 890.1
kJ·mol−1 and second 1980 kJ·mol−1 there is not much that is a reaction partner with gold that is good, it seems as though the only true reaction partners discovered so far, that don’t fuse together to make a compound are Aqua Regia, and Cyanide. But to many scientists dissatisfaction these reaction partner, does not help gold it attacks and breaks down gold. Aqua Regia has been used for centuries as a testing substance for true gold, as obviously it will break true gold down. It’s location on the periodic table is group 11 period 6, which means that gold is a transition metal. Gold’s electron configuration is Xe 4f14 5d10 6s1.
The Bohr Model of Gold and the Lewis Dot Structure of gold are pictured below:
Lewis Dot Structure: Bohr Model:
![]()
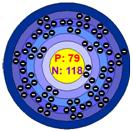

![]()
![]()
![]()
The following are pictures featuring gold:



Bibliography
Gold Project Sources
http://en.wikipedia.org/wiki/Gold
http://hyperphysics.phy-astr.gsu.edu/hbase/tables/sphtt.html#c1
http://www.galmarley.com/framesets/fs_fundamental_properties_faqs.htm
http://wiki.answers.com/Q/What_elements_reacts_with_gold
http://www.chemicalelements.com/elements/au.html
PICS