
Home | About Us | JCPS Home | EHS Science Video
- Chemistry Topics: 1) Matter and Measurement, 2) Atoms, Molecules, and Ions, 3) Stoichiometry, 4) Aqueous Solutions, 5) Thermochemistry, 6) Periodic Properties, 7) Solids, Liquids, and Gases, 8) Chemical Bonding, 9) Molecular Geometry, 10) Properties of Solutions, 11) Chemical Kinetics, 12) Chemical Equilibrium, 13) Acid-Base Chemistry, 14) Thermodynamics, 15) Electrochemistry, 16) Nuclear Chemistry

By Carlton Warren
![]()
![]() It’s not possible to say who discovered it! But Antoine Lavoisier a French chemist discovered that diamonds were a form of carbon. Antoine named carbon and he carried out a variety of experiments to reveal its nature. Carbon has been known since ancient times in the form of soot, charcoal, graphite and diamonds. Ancient cultures did not realize, of course, that these substances were different forms of the same element
It’s not possible to say who discovered it! But Antoine Lavoisier a French chemist discovered that diamonds were a form of carbon. Antoine named carbon and he carried out a variety of experiments to reveal its nature. Carbon has been known since ancient times in the form of soot, charcoal, graphite and diamonds. Ancient cultures did not realize, of course, that these substances were different forms of the same element
![]() BC 3750 Known since ancient times, it was first recognized as an element in the second half of the 18th century.
BC 3750 Known since ancient times, it was first recognized as an element in the second half of the 18th century.
![]() Known since ancient times no exact place found! Carbon is found in abundance in the sun, stars, comets and atmospheres of most planets. Carbon is found free in nature in three allotropic forms; amorphous, graphite and diamond. A fourth form known as "white" carbon is now thought to exist. Graphite is one of the softest known materials, and diamond is one of the hardest. This difference is purely because of the arrangement of atoms in each of the two forms. In graphite, hexagonal rings are joined together to form sheets, and the sheets lie one on top of the other. In diamond, the atoms are arranged tetrahedral (any polyhedron having four plane faces) in a vast continuous array. White carbon is a transparent birefringent material produced during the sublimation of graphite at low pressures.
Known since ancient times no exact place found! Carbon is found in abundance in the sun, stars, comets and atmospheres of most planets. Carbon is found free in nature in three allotropic forms; amorphous, graphite and diamond. A fourth form known as "white" carbon is now thought to exist. Graphite is one of the softest known materials, and diamond is one of the hardest. This difference is purely because of the arrangement of atoms in each of the two forms. In graphite, hexagonal rings are joined together to form sheets, and the sheets lie one on top of the other. In diamond, the atoms are arranged tetrahedral (any polyhedron having four plane faces) in a vast continuous array. White carbon is a transparent birefringent material produced during the sublimation of graphite at low pressures.
![]() Carbon was discovered in prehistory in the form of diamonds, charcoal, and graphite. Carbon is found in combination in hydrocarbons (methane gas, oil, and coal), and carbonates (limestone and dolomite).
Carbon was discovered in prehistory in the form of diamonds, charcoal, and graphite. Carbon is found in combination in hydrocarbons (methane gas, oil, and coal), and carbonates (limestone and dolomite).
![]()
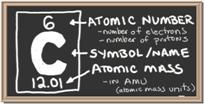
The melting point of carbon is 3500.0 °C, also the boiling point is 4827.0°C. The state of carbon would be listed as a solid, and the density of carbon is g/cm3 (di). The Atomic radio of the carbon is 12.011. The appearance of carbon can be harmful and have harmful effects.
![]()
![]()
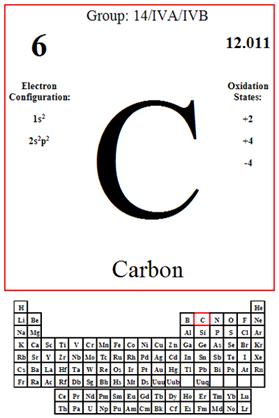
Means the tendency of an atom or radical to attract electrons in the formation of an ionic bond. The electronegative of carbon is 2.55
![]() is the energy required to remove from atom one mole of electrons with subsequent production of positively charged ion of Carbon.
is the energy required to remove from atom one mole of electrons with subsequent production of positively charged ion of Carbon.
![]()
- Carbon is the 4th most common element in the Universe (after hydrogen, helium and oxygen). It is the 15th most common element in the Earth’s crust while it is the second most common element in the human body (behind oxygen).
- The carbon cycle is the process in which carbon is exchanged between all parts of Earth and its living organisms. It is of vital importance to life on Earth, allowing carbon to be continually reused and recycled.

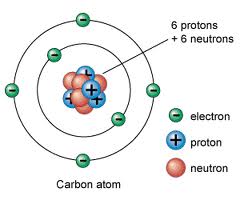
A diamond is forever, unless you heat it too much and it burns up into carbon dioxide gas. Graphite is also pure carbon and widely used in pencils, but not nearly as pretty. In this poster, pretty trumps practical
http://www.google.com/imgres?q=carbon&um=1&hl=en&sa=N&biw=1280&bih=694&tbm=isch&tbnid=_cwKoZTVeHIayM:&imgrefurl=http://periodictable.com/Elements/006/index.html&docid=BxW3k-T60HyFAM&imgurl=http://periodictable.com/Samples/006.x4/s9s.JPG&w=356&h=356&ei=KvuSUP6xKJK89QSy4ICYAw&zoom=1&iact=hc&vpx=474&vpy=211&dur=494&hovh=225&hovw=225&tx=136&ty=110&sig=118353453969561634850&page=3&tbnh=207&tbnw=211&start=20&ndsp=12&ved=1t:429,i:204
http://www.google.com/imgres?q=carbon+on+a+periodic+table&um=1&hl=en&biw=1280&bih=694&tbm=isch&tbnid=BZF_IK5V2MoNLM:&imgrefurl=http://factsontheback.com/chemistry.htm&docid=Ol9jdrzaeV6drM&imgurl=http://factsontheback.com/images/carbon_front.gif&w=400&h=600&ei=GP2SUP-ZCoHk9AS8iIHYCQ&zoom=1&iact=hc&vpx=941&vpy=143&dur=1597&hovh=275&hovw=183&tx=114&ty=111&sig=118353453969561634850&page=1&tbnh=206&tbnw=137&start=0&ndsp=14&ved=1t:429,i:77

http://www.thegeminigeek.com/who-discovered-carbon/
http://www.chemicool.com/elements/carbon.html

