
Home | About Us | JCPS Home | EHS Science Video
- Chemistry Topics: 1) Matter and Measurement, 2) Atoms, Molecules, and Ions, 3) Stoichiometry, 4) Aqueous Solutions, 5) Thermochemistry, 6) Periodic Properties, 7) Solids, Liquids, and Gases, 8) Chemical Bonding, 9) Molecular Geometry, 10) Properties of Solutions, 11) Chemical Kinetics, 12) Chemical Equilibrium, 13) Acid-Base Chemistry, 14) Thermodynamics, 15) Electrochemistry, 16) Nuclear Chemistry
Element Project-Boron(B)

By: Madison Raque
Historical Background- Boron was discovered by French chemists Joseph-Louis Gay-Lussac and Louis-Jacques Thenard. Also, English chemists named Sir Humphry Davy. They all discovered Boron at the same time, but different places. The French team made their discovery in England and Sir Humphy Davy discovered Boron in France of 1808. The element Boron was discovered by isolating boron and combining boric acid with potassium. Today, Boron is obtained by heating borax with carbon.
Physical Properties- Boron is a metalloid; that is hard, brittle and black. It is in a solid state of matter. Its density is 2.34g/cm3 and its melting point is 2075 degrees Celsius. The atomic radius is 98pm and the specific heat is 1.025. Boron represents the majority of the earth’s crust. Also, Boron has never been a free element, but always as a compound in minerals.
Chemical Properties- Electronegativity is the measure of the type of atom to attract a bonding pair of electrons. The scale of electronegativity was developed by Linus Pauling. Boron’s value was 2.04, but electronegativity did not have units. The ionization energy of boron is the energy that must shift from atom one mole of electrons with succeeding production of positively charged ion of boron. Boron reacts with fused sodium hydroxide to form sodium borate and hydrogen. It does not react with the air at room temperature, but at high temperatures it burns to form Boron oxide.
Photographs-
The magnets in a compact flash card hard drive contains neodymium iron boron.

Boron
Periodic Table Element

Silly Putty contains borax and is the key element in keeping its elasticity. Boron never occurs as a free element. It also contains around 4% of boric acid that allows the silly putty to bounce.

Periodic Table-
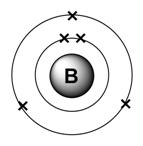
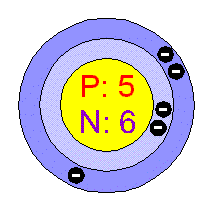
Group: 13
Period: 2
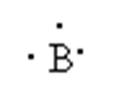
![]()
- The element Boron is not used in many things in your everyday life, but there is one thing that does contains Boron that I use in my everyday life and that is laundry detergent. Boron is a bleach alternative in laundry detergent. This can make your clothes whiter.
Works Cited
Bentor , Yinon. "Periodic Table: Boron." Basic Information. N.p., n.d. Web. 24 Oct. 2012. <www.chemicalelements.com/elements/b.htm>.
Helmenstine Ph. D., Annie Marie. "About.com Search - Find it now!." About.com Search - Find it now!. N.p., n.d. Web. 24 Oct. 2012. <http://cemistry.about.com/od/elementfacts/a/boron.htm>.
"webelement.com." webelement.com. N.p., n.d. Web. 24 Oct. 2012. <http://www.webelement.com/boron/>.
MLA formatting by BibMe.org.
