
Home | About Us | JCPS Home | EHS Science Video
- Chemistry Topics: 1) Matter and Measurement, 2) Atoms, Molecules, and Ions, 3) Stoichiometry, 4) Aqueous Solutions, 5) Thermochemistry, 6) Periodic Properties, 7) Solids, Liquids, and Gases, 8) Chemical Bonding, 9) Molecular Geometry, 10) Properties of Solutions, 11) Chemical Kinetics, 12) Chemical Equilibrium, 13) Acid-Base Chemistry, 14) Thermodynamics, 15) Electrochemistry, 16) Nuclear Chemistry
Barium

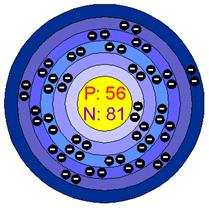
Ba 56
by Kaylee Joyner
Barium is a heavy, silvery-white element that glows red when heated, and keeps glowing for up to an hour after. It’s often mistaken for calcium, and it’s combustible with water, oxygen, and alcohol. When cut, it reacts with the air and turns from silvery white to almost black. Its atomic number is 56, and it conducts electricity very well so is used along with copper in some wires. Its density is 4.50 g/cm3, and it is solid.
Barium’s electronegativity is 0.89, and its atomic weight is 137. It’s located in the 2nd group, 6th period on the periodic table of elements. Barium has a hardness on Moh’s scale of 1.25, and has a boiling point of 1341 °F or 727 °C. The abundance of barium in the earth’s crust is .0425%, and 13 µg/L in sea water. Barium is used to remove unwanted gases from vacuum tubes such as TV picture tubes. It’s used for this because of its low vapor pressure and reactivity towards oxygen, nitrogen, carbon dioxide, and water. Its usefulness is decreasing due to LCD TVs and tubeless plasma sets. It’s used as an alloy with nickel in spark plugs, and is added to steel and cast iron to reduce the size of carbon grains within the microstructure of the metal. Variations of barium are added to fireworks for a green effect.
History of Barium: in the early 1600s, in Bologna, Italy, by Vincentius Casciorolus who was a shoemaker and believed he found the philosopher’s stone. When heated by sunshine, the mineral glows for up to an hour afterwards. When heated, it glowed a bright phosphorous red. Casciorolus was then disappointed when the mineral failed to turn minerals into gold, and when it didn’t make him immortal. Barium was first made known as a mineral by Carl. W. Scheele, a Swedish scientist, in 1774. However, he only isolated barium oxide. He named it “terra ponderosa” (Latin: heavy earth). The element was called barium because it was found in barite (barium sulfate) a mineral given its name because of its high density. The Greek ‘barys’ means heavy. Sir Humphry Davy was the first to isolate barium metal in 1808. Barium is sometimes used now to make glow sticks.
 barium reacting with H2O and O2
barium reacting with H2O and O2
 heated barium
heated barium

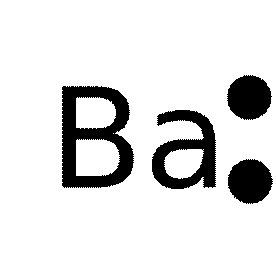 Lewis dot structure of barium
Lewis dot structure of barium
"Barium." Chemicool Periodic Table. Chemicool.com. 15 Oct. 2012. Web. 11/10/2012
<http://www.chemicool.com/elements/barium.html>.
- Kresse, Robert; Baudis, Ulrich; Jäger, Paul; Riechers, H. Hermann; Wagner, Heinz; Winkler, Jocher; Wolf, Hans Uwe (2007). "Barium and Barium Compounds". In Ullman, Franz. Ullmann's Encyclopedia of Industrial Chemistry. Wiley-VCH.doi:10.1002/14356007.a03_325.pub2.
- Lide, D. R. (2004). CRC Handbook of Chemistry and Physics (84th ed.). Boca Raton (FL): CRC Press. ISBN 978-0-8493-0484-2.